The Orange Book and the Purple Book contain valuable information about approved therapeutics in the U.S. Both of these “books” are actually online databases that are routinely used by professionals in the life sciences and biotech industries. Once a therapeutic (small molecule, large molecule, antibody, bispecific, cell therapy, gene therapy, oligonucleotide, etc.) is approved, information about the therapeutic is added to either the Orange Book or the Purple Book. Whether a therapeutic is added to the Orange Book or the Purple Book depends on which legislation governs the approval of the therapeutic.
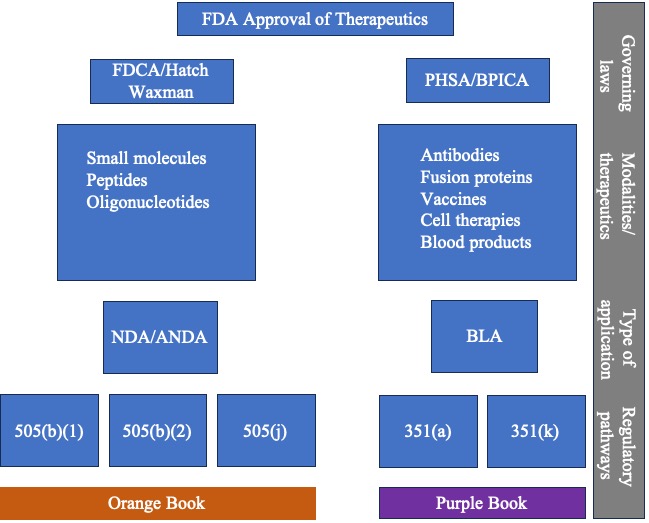
Our previous blog posts covered how the Food and Drug Administration (FDA) came to approve therapeutics under the passage of various laws by Congress. We have covered the Federal Food, Drug, and Cosmetic Act (FDCA) of 1938, the Public Health Service Act (PHSA) in 1944, the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Amendments, and the Biologics Price Competition and Innovation Act of 2009 (BPCIA). We also covered new drug applications (NDAs), abbreviated new drug applications (ANDAs), and biological license agreements (BLAs) and the various approval pathways (505(b)(1), 505(b)(2), 505(j), 351(a), and 351(k)) under these laws. Below is a diagram that summarizes the relationship between these laws, modalities, type of applications, and approval pathways.
The Orange Book
Therapeutics approved under the FDCA and Hatch Waxman amendments are listed in the Orange Book. Therapeutics that have not been approved by the FDA, such as therapeutics in clinical trials, are not listed in the Orange Book. It is called the “Orange Book” because early publications were in a book form and the front cover was orange. However, its formal name is “Approved Drug Products with Therapeutic Equivalence Evaluations,” and it is now an online resource found here. It is a compilation of therapeutics approved by the FDA under the FDCA.
The Orange Book contains important information, including whether a drug is approved under an NDA or an ANDA. For example, consider the drug or active ingredient plerixafor. A search of the Orange Book for this active ingredient yields the following search results:
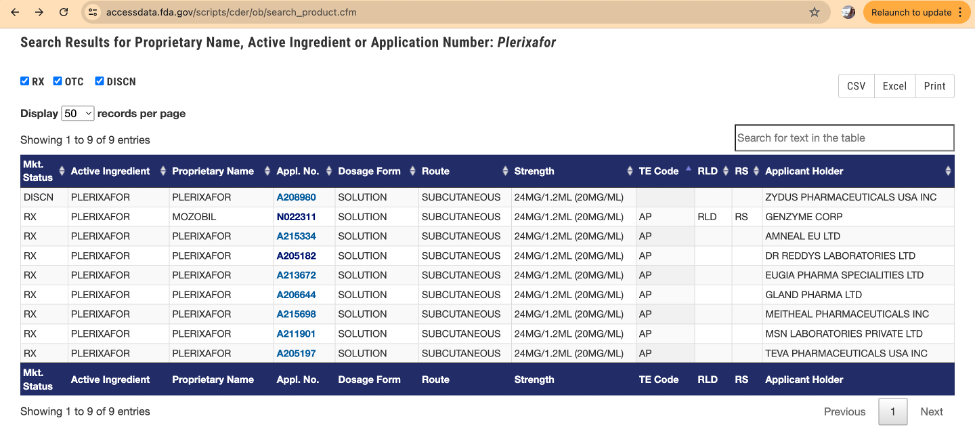
Under the column “Appl. No.”, the approved NDAs (denoted with an “N” application number) and the approved ANDAs (denoted with an “A” application number) are listed. For each of these applications, the applicant holder is also provided. For this drug or active ingredient, there is an NDA holder (Genzyme Corp.) and several ANDA holders.
For comparison, consider a search in the Orange Book for the active ingredient pirtobrutinib:
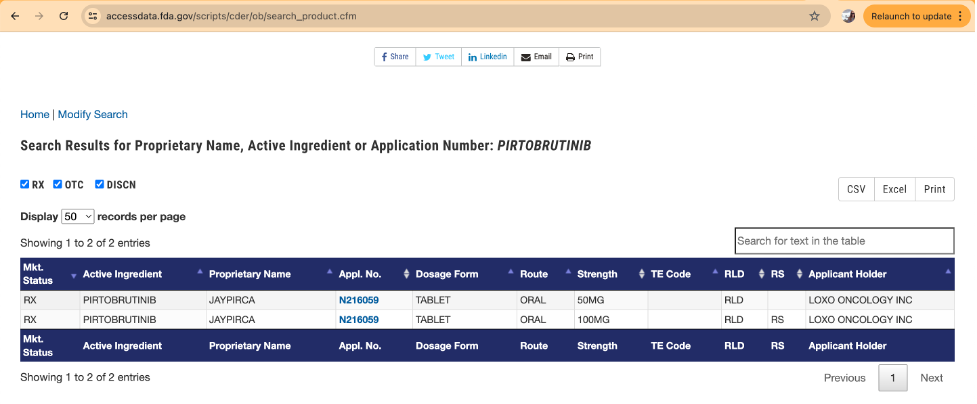
For pirtobrutinib, there is only one approved NDA, which covers two different strengths of tablets (50 mg and 100 mg). There are no approved ANDAs for this active ingredient. Also notice how the active ingredient name differs from the proprietary name. The active ingredient name is the name of the actual molecule that provides the pharmacological activity or effects the treatment or prevention of the disease. “Active ingredient” means the particular molecule that causes an effect in humans. The proprietary name, also known as the brand name or trade name, is the name that will be used in the marketplace for the particular drug product. For example, “aspirin” is the name of a specific molecule or active ingredient. “Bayer” is the proprietary name or brand name of the drug product marketed and distributed in the marketplace.
As we discussed in our previous blog post Therapeutics at the FDA: paths to approval, the FDA will not require an applicant to repeat all the pre-clinical and clinical studies of a drug that has been previously approved by the FDA. These generic drugs are approved under 505(j) via an ANDA. In the Orange Book, the FDA will indicate the active ingredient that can serve as a reference drug for an ANDA. These active ingredients are noted as the RLD (Reference Listed Drug). The RLD is the reference standard by which any ANDA applicant must use in its testing to establish the generic product’s bioequivalence or “sameness.” The Orange Book also indicates the Reference Standard (RS). The reference standard is the drug product selected by FDA that an applicant seeking approval of an ANDA must use in conducting an in vivo bioequivalence study required for approval of an ANDA. If there are different strengths of a dosage form, the FDA usually selects the highest strength as the RS. Notice that in both of our examples, plerixafor and pirtobrutinib, one NDA serves as the RLD. In the pirtobrutinib example, the highest strength dosage form is designated as the RS.
The Purple Book
Therapeutics approved under the PHSA and the BPCIA are listed in the Purple Book. The official name is “Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations,” and it made its debut on the FDA’s website on September 9, 2014. It was given the informal name the “Purple Book” to be similar to the informal name of the “Orange Book.” Thus, we have the Orange Book and the Purple Book in the United States to list approved therapeutics.
The Purple Book contains information about all FDA-licensed biological products, including licensed biosimilar and interchangeable products, and their reference products, in addition to FDA-licensed allergenic, cellular and gene therapy, hematologic, and vaccine products. Similar to the Orange Book, only approved therapeutics are listed. Therapeutics in the clinic or not yet approved by the FDA are not listed in the Purple Book.
As an example, consider a search in the Purple Book for “aflibercept” that yields the following results:
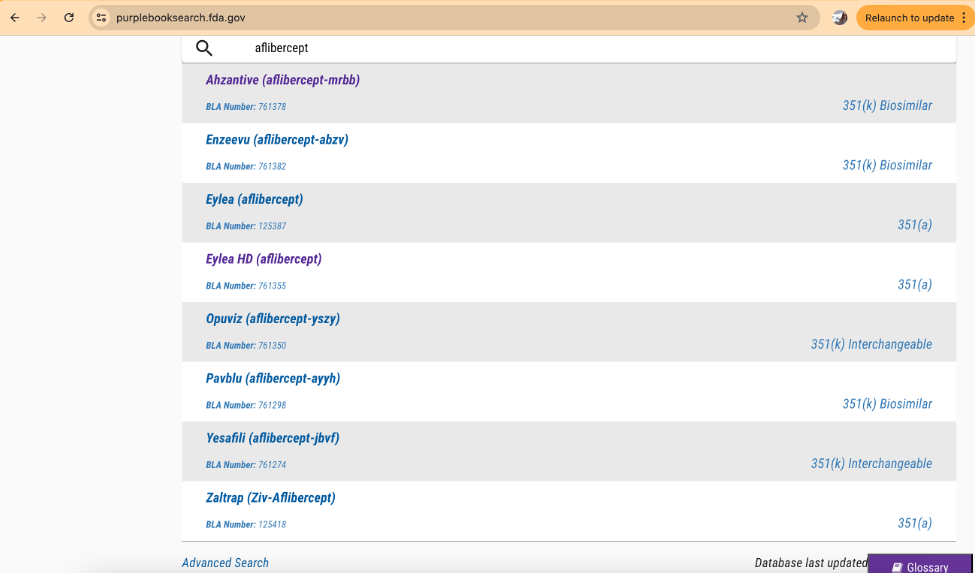
In the right column, the Purple Book provides the regulatory pathway (351(a) or 351(k)) for the drug product. For 351(k) approvals, it also specifies whether the product is a biosimilar or interchangeable.
In the left column, the proprietary name and the proper name for each product is provided. The proper name, or active ingredient name, is shown in parenthesis. For biologics, a random, four-letter suffix is added to the proper name. This additional naming convention is to facilitate accurate identification of biological products by health care practitioners and patients. It also helps minimize inadvertent substitution of products that have not been designated as interchangeable.
For aflibercept, there are approved reference products (approved under 351(a)), along with biosimilar and interchangeable products (approved under 351(k)).
In contrast, a search of the Purple Book for epcoritamab yields the following results:
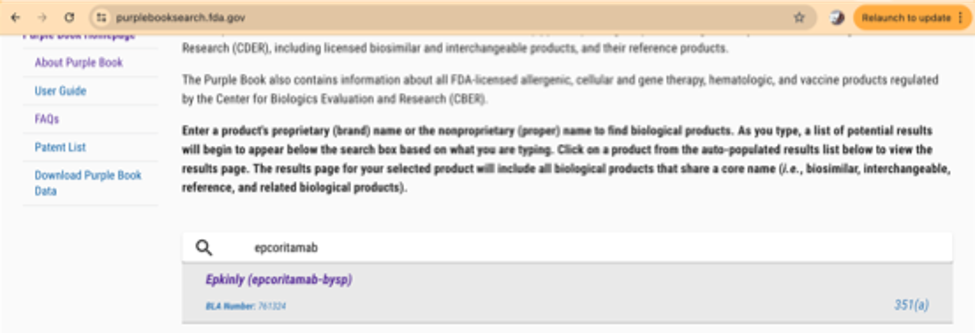
For this active ingredient (epcoritamab-bysp), there is only one approved product, marketed under the brand name Epkinly.
The Purple Book and Orange Book serve as valuable resources for professionals in the life sciences or biotech industries. However, each of these online resources are limited to approved therapeutics. Therapeutics currently in the clinic are not listed in either online resource.
For a comprehensive guide to the different therapeutics and modalities, Science for Bankers’ Executive Course: Therapeutics & Modalities provides another valuable resource to professionals in life sciences and biotech. This video-based course provides the necessary scientific background needed for professionals working in life sciences or biotech.